diseig rc2h2ph_s_d > rc2h2ph_s_d_diseig.log
rc2h2ph_s_d_diseig.log, contains diagnostic messages,
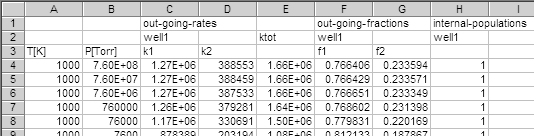
rc2h2ph_s_d_diseig_hpl.csv contains the high-pressure limiting
rate coefficients, and the file, rc2h2ph_s_d_diseig_out.csv,
contains the essential results of calculations, part of which is shown below:
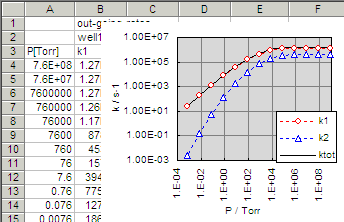
k1 and k2 shows the rate coefficients
for the thermal decomposition of 2-phenylvinyl radicals to phenylacetylene + H
(k1) and phenyl radical + C2H2
(k2), respectively. As described in the previous
example, the channels are ordered from one with lowest E0
to higher. The fall-off pressure dependence of the rate coefficients
looks like: