thermEquil ctr_equil.inp
ctr_equil.csv.
The contents should be:
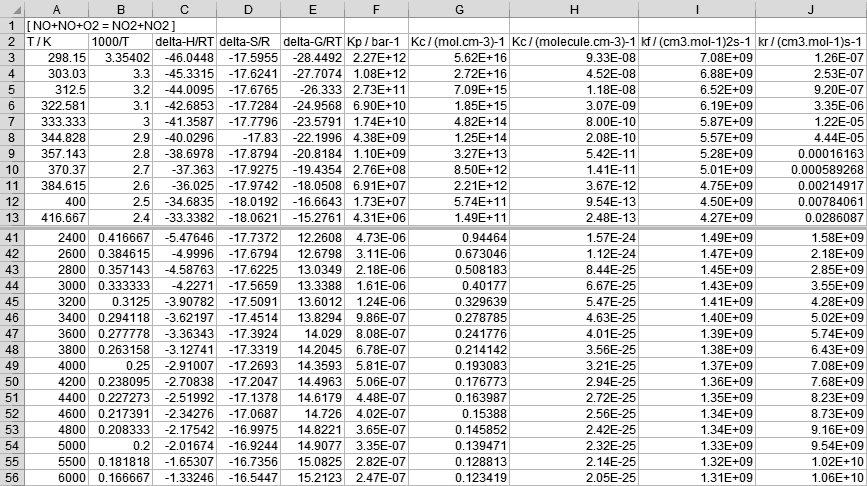
Following is an example of the calculation of thermodynamic properties and equilibrium constants of a specified reaction for versatile list of temperatures. The example also contains the calculation of reverse rate constants.
Control input
ctr_equil.inp to
specify the list of temperature and the thermodynamic data file as
well as the reaction to be investigated.
The contents are:# sample control input for thermEquil tempList 298.15 tempRecipRange 10000 11 33 1 tempRange 1000 2300 100 tempRange 2400 4800 200 tempRange 5000 6000 500 thermodata dataNOx.dat reactants NO NO O2 products NO2 NO2 forward_k mol/cm3 cal/mol 1.19678e+09 0 -1053.22
'temp' specify the list
of temperatures and 'thermodata' input specifies the
thermodata file name and its format (new or old).
Since old is default, it was omitted in this example.
'reactants' input specifies the reactants with the name
registered in the thermodata file. Similarly, 'products'
specifies products.
'forward_k' specifies the
forward rate constant and program will calculate the reverse rate constants.
Without 'forward_k', the program just calculates the
equilibruim constants (not reverse rate constants).
'forward_k' specify the unit systems
of concentration (1st argument) and activation energy (2nd).
Valid unit system for concentrations are mol/cm3 or
molecule/cm3 while they are cal/mol or
kelvin for activation energy. The remaining arguments are
parameters for modified Arrhenius expression,
A, n, and Ea.
Execute thermEquil
ctr_equil.csv.
The contents should be:
