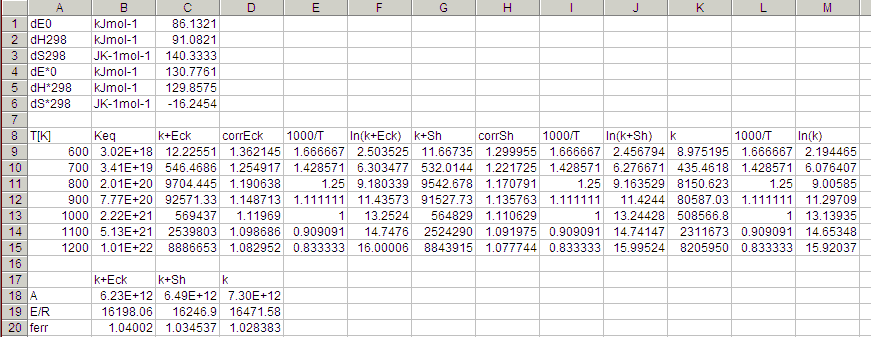
basename.csv. An output from the sample input
EtO2concHO2elim.rxn is shown below.
Synopsis
Description
tstrate reads a reaction input file,
basename.rxn, and GPOP-format files for molecules
or atoms described in the reaction input.
Rate constants and/or equilibrium constants are calculated and results
are written in a CSV file, basename.csv.
gpop1scf reference manual. However, the imaginary frequency
for the tunneling correction, it is used UNSCALED.
Input
| 1) | A reaction input file, basename.rxn. | |
| 2) | GPOP-format files (*.gpo). | |
Note: The .gpo files should be pre-processed by
gpop3tst before using tstrate.
Even in the case that no modification is needed, it must be
pre-processed with a null .mod file.
The energetics is the one of the most important property in the
rate constant calculation.
The energy of the reactants and the TS (and/or the products)
must be specified either explicitly in the .rxn
file, or should have been specified by energyTST
in the .mod file.
| ||
basename.rxn,
should contain a reactants block and at least one of a
products block or a transitionState block.
If a reactants and a products block are found,
the program calculates the equilibrium constants for the reaction.
An example reaction input for calculating equilibrium
constants for C2H5 →
C2H4 + H is shown below.
reactants{
file ethyl500
}
products{
file etln500 hatom50
}
Each block should contain at least one file key, specifying
the '.gpo' file(s) to be read.
If a reactants and a
transitionState block are found, the program calculates the
rate constants for the reaction. If all three blocks, reactants,
transitionState, and products blocks, are found,
the rate constants as well as the equilibrium constants are calculated.
Since the tunneling correction to the rate constants using
an asymmetric Eckart potential requires the energy for products,
this correction is only made when all three blocks are found.
An example of a reaction input with three blocks is shown below.
reactants{
file etp500
}
transitionState{
file tsho501
}
products{
file hoo500 etln500
}
All valid keys in the blocks or the outside the blocks will be described
in detail below.
energyUnit unit
energy key in the blocks.
Note that this NEVER override the unit of energyTST key
in '.gpo' files, which is always hartree.
Values may be one of 'hartree' (default),
'kJ/mol', and 'cm-1'.
tempRange T_start T_end T_step tempRecipRange numer start end step tempGauChebGrd T_min T_max nT tempList T1 T2 T3 ...
tempRange or
tempList can be used. Same as these keys in the
temperature file format in
gpop4thf.
fileType ftyp
gpo' (default) and 'tst'. This is retained for
older compatibility.
energy engy
energyUnit' key outside the block.
imagFreqTS imgfrq
Output
basename.csv. An output from the sample input
EtO2concHO2elim.rxn is shown below.

References
| [4] | B. C. Garrett and D .G. Truhlar, J. Phys. Chem. 83, 2921 (1979). |
| [5] | I. Shavitt, J. Chem. Phys. 31, 1359 (1959). |